Medical Device Regulation 2022: What’s the latest?
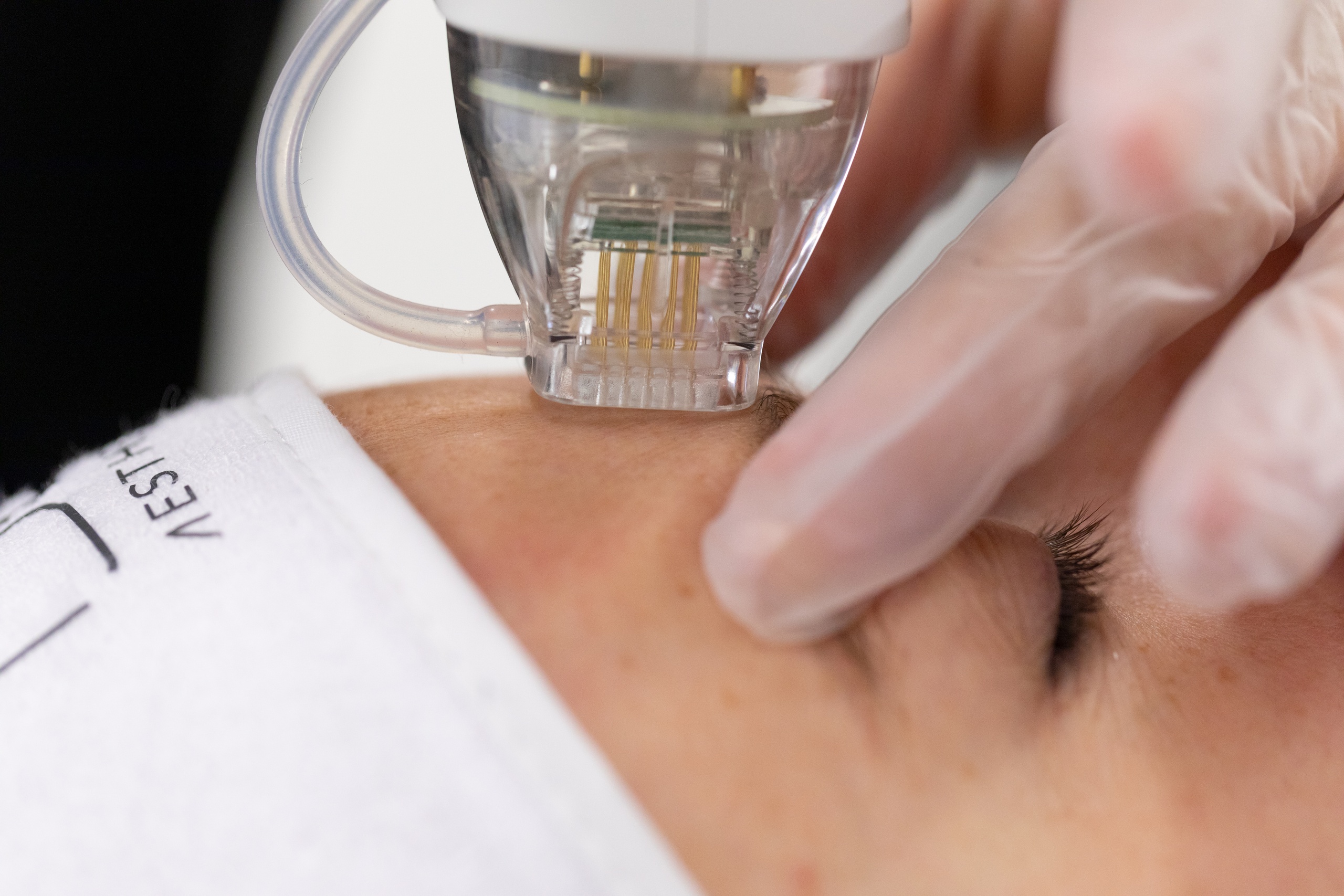
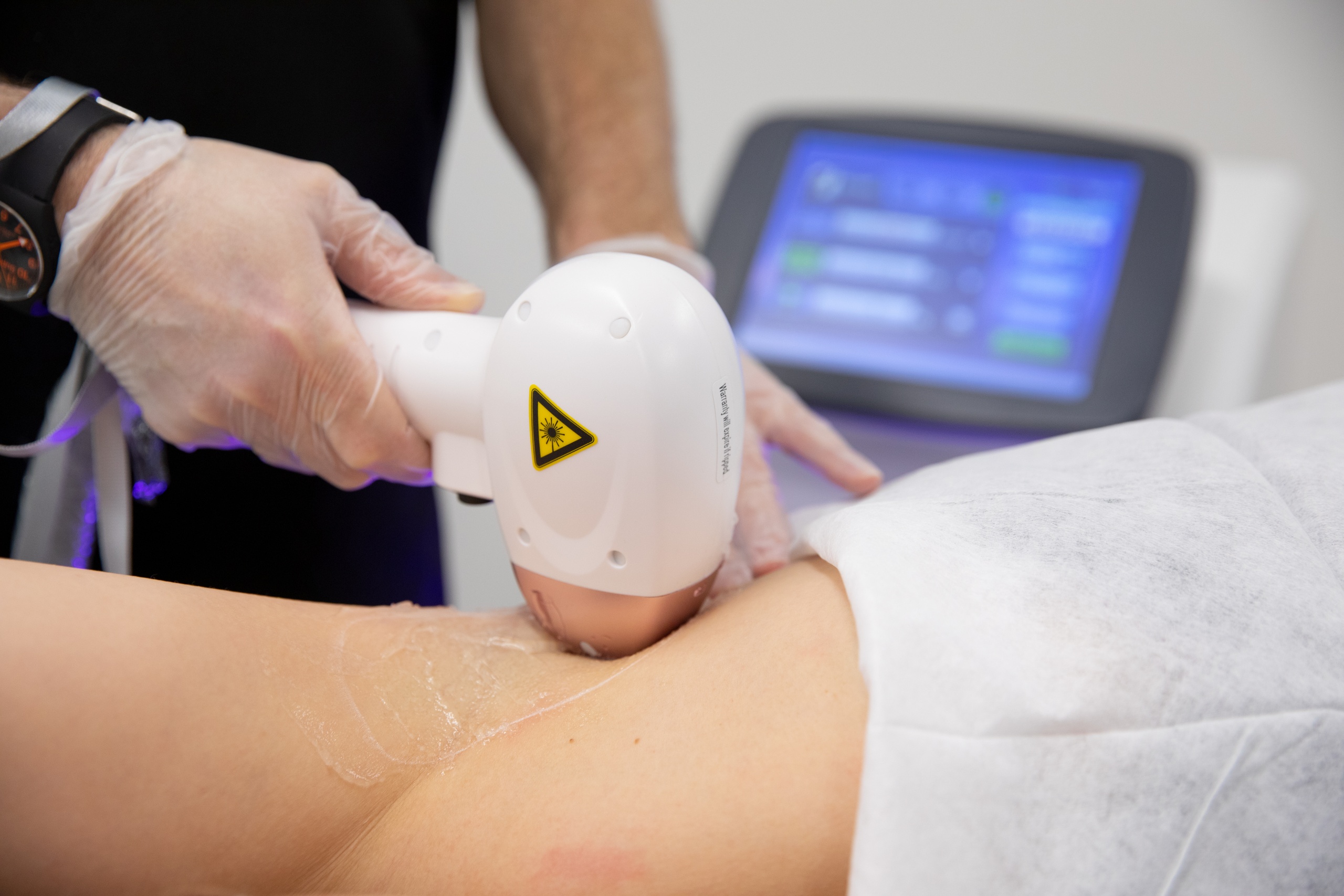
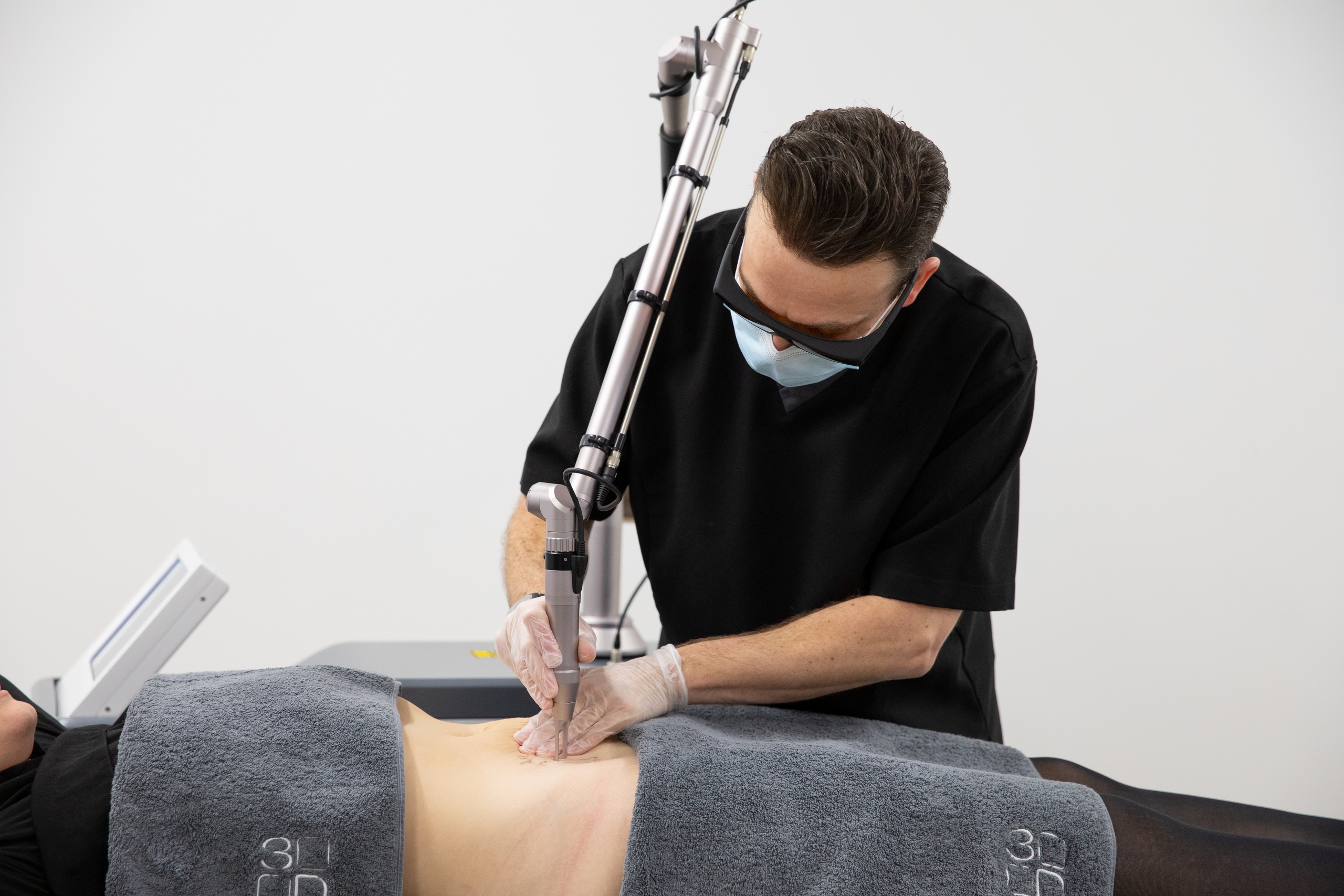
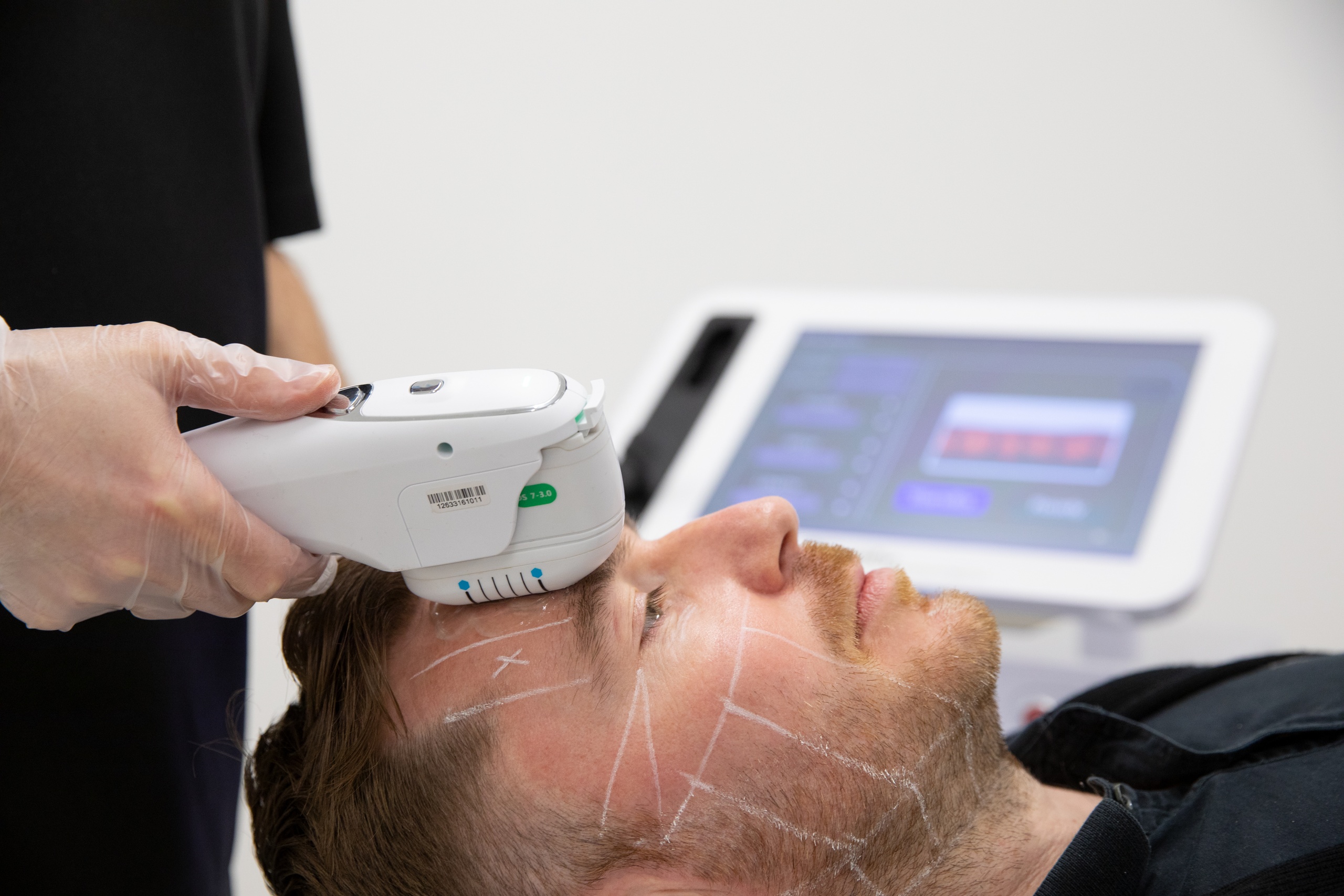
New regulations could mean that devices used for treatments such as microneedling, non-invasive fat removal, radiofrequency, filler, LED, lasers and IPL will be subject to medical device regulation.
There is significant confusion surrounding the regulations and certification of medical and aesthetic devices in our industry from both suppliers and customers.
In this article, I will aim to update on the latest regulations and clarify what is coming down the line, to ensure that salon and clinic owners can purchase devices with confidence.
The new Medical Device Regulation (MDR) was first introduced on May 25, 2017, marking the start of the three-year transition period from the Medical Device Directive 93/42/EEC (MDD), for manufacturers selling medical devices in Europe.
At the time, manufacturers were given three years from when the MDR was published to update their technical documentation and processes in order to meet the new requirements.
The new regulations were due to come into force in May 2020, and we would have seen a significant change in our industry. I estimate that at least 80% of the relevant device suppliers would not have been able to upgrade their certification or afford the investment to do so.
However, before the regulations came into play, Covid happened and the Government delayed the implementation of the new directive. As the threat of Covid eased, we left the EU, which totally transformed the legislative landscape.
The difference between what constituted a medical device in the MDD and what counts as one in the new MDR is quite different – here’s what you need to know.